Graham-Field produces most of our products at our wholly-owned advanced manufacturing facilities in Atlanta, GA; Warwick, RI; High Point, NC; and Fond-du-Lac, WI. Our growing Made in USA program provides customers with quick access to top-quality Stretchers, Procedure Chairs, Beds, Recliners, Support Surfaces and an extensive Bariatric Patient Care equipment line. Significant investments in infrastructure expansion, fiber optic laser cutting systems, cobots, innovative powder coating technology, nitrogen production, robotic welders, computer-assisted sewing machines, compression packaging systems, nano technology water filtration systems, and CNC cutters, our goal is to continue to expand our US production to make quality products closer to our customers and supports American jobs.
Made in the USA

ISO 13845 and MDSAP Certified
We take pride in being an ISO 13485:2016 / MDSAP certified manufacturer, accredited by Intertek. Achieving and maintaining this certification has been a fundamental objective for our business as we expand in the medical device industry, ensuring our commitment to delivering high-quality products to our customers.
What is the ISO 13485 Standard?
The ISO 13485 Standard is an International Standard which specifies requirements for a quality management system (QMS) specifically for organizations involved in the design, production, installation and servicing of medical devices. Complying with the ISO 13485 standard helps organizations establish a Quality Management System (QMS) that will let them build and sustain effective methods and processes in manufacturing medical devices. By having this certification, we are committing to align our quality standards to internationally recognized guidelines to ensure top-notch and streamlined systems in designing, producing, and distributing safe and high performing medical devices.
Some areas the ISO 13845 standard focuses on are:
- Detailed quality management system
- Defining and adhering to processes & procedures for controlling documentation and records
- Ensuring design and development of medical devices under a detailed quality system
- Supplier Controls and Aggressive Factory Inspection Programs
- Setting in place controls and processes for identification and traceability of our medical devices and its components.
- Rigorous Product Testing
What is MDSAP?
The Medical Device Single Audit Program (MDSAP) is a program that allows a single audit to satisfy the requirements of multiple regulatory authorities, including those in the US (FDA), Canada, Brazil, Japan and Australia.
What are the Benefits of our ISO 13485/MDSAP Certifications to YOU our Customer?
These certifications require us to operate and adhere to our processes and procedures on a daily basis. For our customers it means:
- Streamlined processes: The programs help organizations streamline their quality management systems, potentially reducing time and resources spent on compliance. This efficiency can lead to quicker product launches and increased sales velocity.
- Enhanced Credibility: The ISO 13845:2016 and MDSAP certification demonstrates our commitment to quality and regulatory compliance to industry standards and best practices for our customers
- Regulatory Compliance: Being certified helps ensure compliance with relevant regulations, reducing the risk of costly penalties and enhancing market reputation.
- Market Access: By complying with MDSAP requirements, companies can facilitate easier entry into multiple markets, including the US, Canada, Brazil, Japan and Australia. This broadens potential sales opportunities.
- Product Improvements: By committing to having a certified Quality Management System we use customer feedback, quality reporting, trending and analysis to constantly monitor the quality of our products and implement improvements as needed.
- Top notch service levels: Maintaining compliance allows us to keep manufacturing and quality as our top priority to deliver not only superior products but also the service needed to support you as our customer.

At our Atlanta facility, we manufacture high-quality clinical recliners, support surfaces, bariatric wheelchairs, and patient slings. Our 120,000 sq ft facility invests in expert staff and equipment.
Skilled workers meticulously transform materials into vital medical equipment for healthcare facilities nationwide. We use premium components and rigorous quality control for low return rates.
Graham-Field blends craftsmanship and experience to make durable equipment we proudly stand behind.
Made In Atlanta

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
Since 1940, we’ve proudly manufactured American built beds in Fond du Lac, WI. Now in our 400,000 sq ft plant, we are dedicated to delivering not only top-quality long-term / homecare beds, but also Hausted Stretchers and Procedure Chairs, Gendron branded Bariatric line of products and our Transfer Master line of Consumer Beds.
High-grade steel undergoes precise bending/stamping and laser tube cutting for uniform framing. Robotic welding bonds large assemblies. Anti-microbial coatings protect frames. Rigorous processes ensure uncompromising quality in every bed and stretcher produced.
Made In Fond du Lac



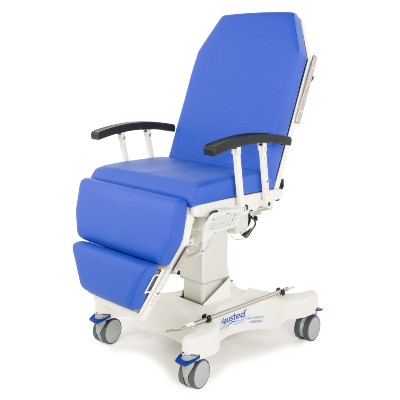

Our High Point, NC facility has manufactured Hausted exam/lab furnishings since 2018. This 40,000 sq ft plant's staff make high-quality products using premium frames, foam and healthcare fabrics for superior comfort, function and durability.
Upholstery and assembly exhibit skilled artistry. With a complete line of furnishings, each piece offers useful features designed to provide solutions and add aesthetic appeal for any area.
Made In High Point







Graham-Field's Warwick, RI facility produces two essential medical products: Silver Nitrate and Unna's boot bandages. These items play a crucial role in daily primary and acute care settings.
Silver nitrate, a topical agent, is applied in wound management to ward off infections and enhance the healing process. Unna boots, specialized compression dressings, are employed to address various leg conditions, with a focus on managing swelling and edema.


By manufacturing these vital medical supplies domestically, Graham-Field helps ensure consistent availability for patient care across the United States healthcare system.
Made In Warwick













